1.Causes of corrosion of electric furnace lining during normal use
During normal smelting, the furnace lining is in direct contact with high-temperature molten steel and slag, and the working conditions are very harsh. The reasons for furnace lining damage are:
(1) Thermal spalling caused by arc radiation and chemical erosion at high temperatures.
(2) The scouring effect of molten slag, molten steel and furnace gas on the furnace lining.
(3) Chemical erosion of the furnace lining by molten slag.
(4) Peeling caused by temperature changes.
(5) Cracking caused by the decomposition of the mineral composition of the furnace lining brick itself.
(6) Mechanical collision and erosion of the furnace lining when adding scrap steel and adding molten iron.
2.The erosion process of magnesia carbon bricks during electric furnace smelting
Magnesia carbon bricks are made of dead-burned magnesia or fused magnesia and carbon materials (mainly fully crystallized graphite), prepared and pressurized with resin as a binder, and formed after heat treatment. In order to improve the oxidation resistance, metal or other antioxidants are often added to magnesia carbon bricks. Magnesia carbon bricks are generally used as refractory materials for electric furnace walls.
The basic process of magnesia carbon brick erosion during the use of electric furnaces :
(1) Magnesia carbon bricks are divided into three layers after reaction: original brick layer (the brick body that has not reacted) – decarburization layer (the internal MgO and carbon undergo a self-consumption reaction) – dense layer (the part in contact with steel slag).
(2) The MgO and carbon inside the magnesia carbon brick undergo a self-consumption reaction at high temperature:
MgO+C=Mg+CO
Mg+[O]=MgO
(3) The oxides in the slag react directly:
(Fe2O3)Slag+C Brick=2(FeO)+{CO}
Magnesium oxide is reduced to magnesium gas and migrates along the pores of the refractory material to the surface where it is secondary oxidized to MgO, and forms a high-viscosity petrographic structure with other impurity elements in the middle of the brick, which is commonly known as a dense layer. The erosion processes are as follows:
(1) Physical wear and tear.
During the blowing process of the converter, the movement of the physical steel slag furnace gas causes physical and mechanical wear of these petrographic structures to peel off and enter the slag.
(2) Chemical attack.
The chemical reaction is that various components in the middle of the slag will react with the dense layer of the bricks. FeO can promote the dissolution and transfer of magnesia to the middle of the slag and increase the erosion of magnesia carbon bricks.
(3) The effect of temperature on erosion.
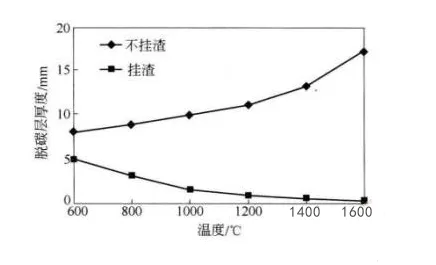
The higher the temperature, the viscosity of steel slag decreases, physical erosion intensifies, and the decarburization layer deepens, causing erosion to intensify.
3.The loss mechanism of magnesia carbon bricks lined with electric furnaces
Generally, magnesia carbon bricks are made of high-quality magnesia with high-purity graphite, silicon, silicon carbide and other additives, and are pressed with phenolic resin as a binder. The basic requirements for magnesia carbon bricks for electric furnaces are:
(1) The thermal conductivity is low to ensure less heat loss and improve the thermal efficiency of the electric furnace;
(2) High resistance to thermochemical and thermophysical corrosion coefficients, which requires good volume stability;
(3) Resistance to slag, peeling, oxidation and high compressive strength, resulting in low consumption and long life.
When baking a new furnace lining, the following main reactions will occur when the furnace lining temperature reaches 750°C:
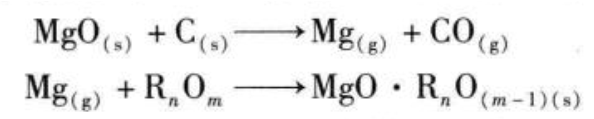
The above reaction 1 mainly involves the generated magnesium gas and carbon monoxide gas migrating to the high temperature area along the pores. The reaction 2 is that the magnesium gas on the surface of the furnace wall is oxidized again by the terbium compound into magnesium oxide, and forms a high melting point with other trace compounds in the magnesia carbon brick. of petrographic compounds. Therefore, controlling the temperature system of the oven and preventing a large amount of reaction 1 is the key to maintaining the volume stability of magnesia carbon bricks. This is very important whether in a converter or an electric furnace. The direct consequence of the failure of the oven is the collapse of the furnace lining, or the service life of the furnace lining is greatly reduced. Most domestic manufacturers have already had a lot of experience and lessons on this.
